Go back to RESOURCES
Applications For The C-Pace System
5 years of the C-Pace: a short review
Cardiac tissue * Skeletal muscle * Stem cells * Cell-lines * Cardiogenic Fibroblasts * Neuronal fibroblasts * Osteoblasts
It has been more than 5 years since Ionoptix brought a device to the scientific research community that allowed chronic electrical stimulation of cells in culture. Since the introduction of the C-Pace, researchers have found a wide range of applications for it, for many different cell types. Here we provide a brief review of the history of the chronic electric stimulation of cells in culture, the applications it is being used for and citations for the relevant papers.
History
We started working on a device for chronic electrical stimulation of cells in culture after requests from several cardiac research groups. A paper by the group of Ralph Kelly and Thomas Smith (Berger, et al. 1994) showed the benefits of pacing adult cardiac myocytes in culture. Mechanical activity of myocytes in culture allowed them to retain their phenotype while retaining their contractile characteristics. This work was elaborated on in later years by the group of George Cooper and Paul McDermott. They showed that chronic pacing not only allowed the retention of phenotype (Kato, et al. 1995) but also enhanced protein synthesis (Ivester, Kent, et al. 1993)(Ivester, Tuxworth, et al. 1995). Based on their work and ideas we decided to develop a commercially available multi-channel pacer.
Cardiac muscle; adult, neonatal, embryonic and cell lines
Early users of the C-Pace confirmed the usefulness of the C-Pace unit for myocyte culture. It can be used to establish a stable primary culture that, for example, allows expression of proteins using adenovirus transfection while maintaining the ability to study excitation-contraction coupling (Mills, et al. 2006)(Ahlers 2005). The chronic pacing of cells does alter signaling pathways favorably but can also predispose a fraction of the cells to apoptosis (Kuramochi, Guo, et al. 2006)(Kuramochi, Lim, et al. 2004). It is possible that the inflexible surface of culture dishes causes a problem. A future development could be the use of flexible substrates to better mimic physiological conditions.
Apart from chronic stimulation, the system has also been used for short-term bulk stimulation of cultured cells to achieve a variety of effects. Examples are to achieve the translocation of calmodulin kinase (W. Thiel, et al. 2008)or transcription factors from the cytoplasm to the nucleus (Schroder, Byse and Satin 2009). Another report indicates that chronic pacing during virus transfection increases transfection efficiency (Rana, et al. 2008). Increasing the mechanical activity with pacing induces elevated myocyte metabolism. Field stimulation of large quantities of cells has been shown to be a useful tool in the study of myocyte metabolism (Luiken, et al. 2001).
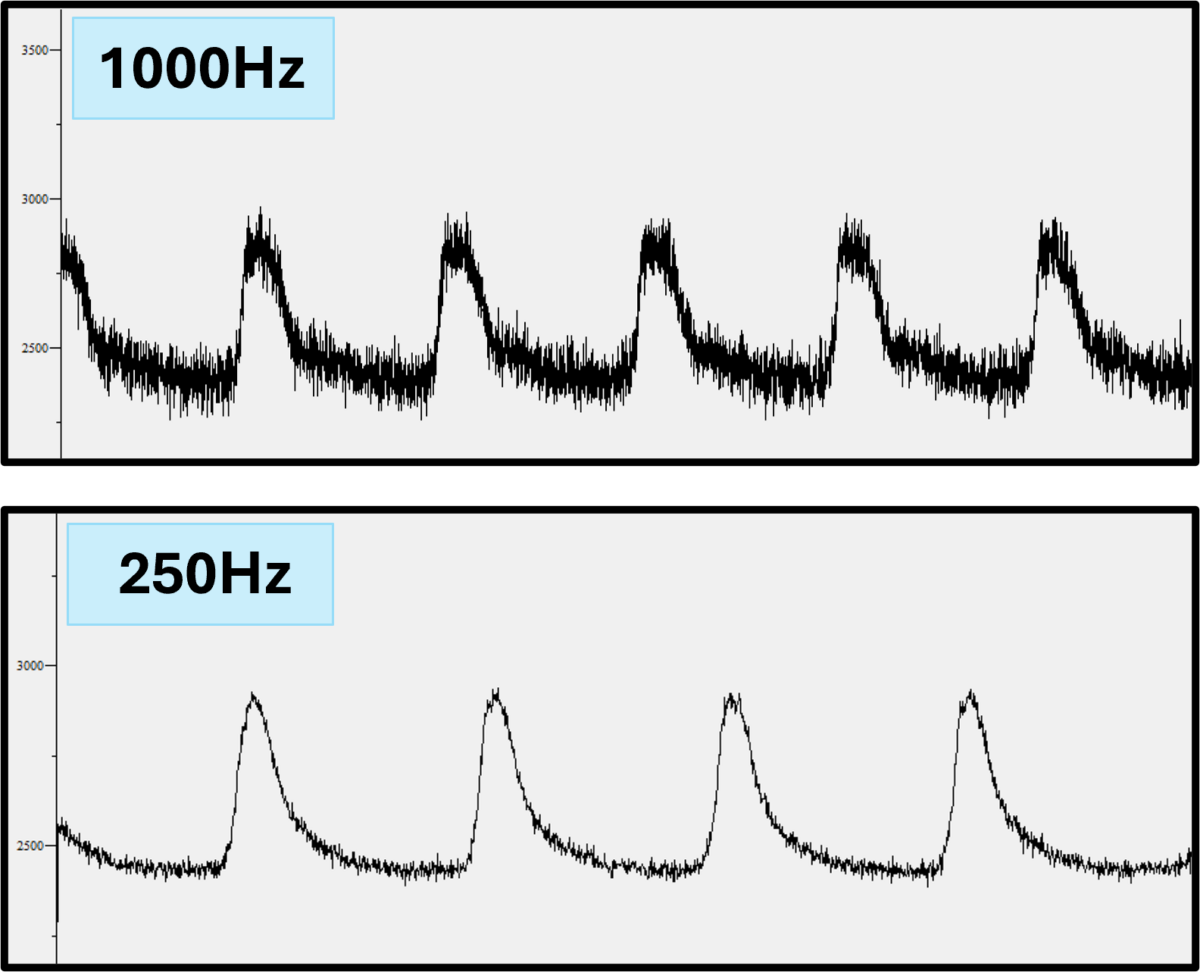
Interesting studies have been performed using the C-Pace system to mimic tachycardia. Brundel et al used it to look at stress responses in HL1 cell lines upon induced tachycardia (Brundel, Henning, et al. 2006)(Brundel, Shiroshita-Takeshita, et al. 2006). The group of S. Nattel has published a very elegant study showing electrical remodeling in cultured canine myocytes with high frequency pacing over a 24 hour period (Qi, et al. 2008). This is one of several studies to show that pacing exerts its initial effect by activating L-type calcium channels and thus elevating intracellular calcium levels. L-type calcium channels are obviously important for calcium homeostasis in adult cardiac cells, but also suggest that pacing can be used to great effect on non-excitatory cells (see below).
Cardiogenic fibroblasts, stem cells and bone marrow cells
The work of Genovese and colleagues show the potential of electrostimulation for stem cell differentiation and provides clues why electrostimulation has such a strong effect on muscle phenotype. In an initial study the group showed that electrostimulation can induce cardiac pre-commitment of stem cells or fibroblasts (J. Genovese, et al. 2008). It possibly does so by the upregulation of follistatin which ‘is considered to be a key modulator in muscle development, differentiation and regeneration’ (J. Genovese, et al. 2009).
Skeletal muscle
The C-Pace has been used successfully on skeletal preparations. A couple of papers have been published employing C2C12 cell lines, where pacing can be used to induce de-novo sarcomere formation in incompletely differentiated myotubes (Fujita, Nedachi and Kanzaki 2007). Alternatively, in fully differentiated myotubes, stimulation can be used to achieve a hypertrophic response, rather similar to the signaling programs seen in intact muscle upon exercise (Nedachi, Fujita and Kanzaki, American Journal of Physiology- Endocrinology And Metabolism 2008)(Nedachi, Hatakeyama, et al. 2009).
Other uses; nerve cells and osteoblasts
The potential scope for chronic pacing is almost limitless as many cell types contain voltage sensitive channels. In neuronal fibroblasts electrical stimulation can be used to modulate the differentiation of embryonic stem cells to a neurological phenotype (Yamada, et al. 2007). The C-Pace system has been used in neuronal fibroblasts to induce expression of the NMDA receptor by activating L-type calcium channels (Okutsu, et al. 2008). Another application is in the culture of osteoblasts, where low level currents can help with the attachment to artificial surfaces and enhance proliferation of osteoblasts (Ercan and Webster 2008).
Future
The list of uses for the C-Pace system will likely expand as it is applied to many different types of experiments. From our end, we will strive to keep improving the design of the C-Pace and the C-Dishes to support ongoing research and new uses. The device was developed in close cooperation with the scientific community, and we will try to keep the communication going, so that we can improve the design according to your needs.
Works Cited
Ahlers, B. A. “Effects of sarcoplasmic reticulum Ca2+-ATPase overexpression in postinfarction rat myocytes.” Journal of Applied Physiology 98, no. 6 (Feb 2005): 2169-2176.
Berger, H J, et al. “Continual electric field stimulation preserves contractile function of adult ventricular myocytes in primary culture.” The American journal of physiology 266, no. 1 Pt 2 (Jan 1994): H341-9.
Brundel, Bianca J J M, et al. “Induction of heat shock response protects the heart against atrial fibrillation.” Circulation Research 99, no. 12 (Dec 2006): 1394-402.
Brundel, Bianca J J M, Robert H Henning, Lei Ke, Isabelle C van Gelder, Harry J G M Crijns, and Harm H Kampinga. “Heat shock protein upregulation protects against pacing-induced myolysis in HL-1 atrial myocytes and in human atrial fibrillation.” Journal of Molecular and Cellular Cardiology 41, no. 3 (Sep 2006): 555-62.
Ercan, Batur, and Thomas J Webster. “Greater osteoblast proliferation on anodized nanotubular titanium upon electrical stimulation.” International journal of nanomedicine 3, no. 4 (Jan 2008): 477-85.
Fujita, Hideaki, Taku Nedachi, and Makoto Kanzaki. “Accelerated de novo sarcomere assembly by electric pulse stimulation in C2C12 myotubes.” Experimental cell research 313, no. 9 (May 2007): 1853-65.
Genovese, J, C Spadaccio, J Langer, and J Habe. “Electrostimulation induces cardiomyocyte predifferentiation of fibroblasts.” Biochemical and Biophysical Research Communications, Jan 2008.
Genovese, Jorge A, Cristiano Spadaccio, Hernan Garcia Rivello, Yoshiya Toyoda, and Amit N Patel. “Electrostimulated bone marrow human mesenchymal stem cells produce follistatin.” Cytotherapy 11, no. 4 (Jan 2009): 448-56.
Ivester, C T, et al. “Electrically stimulated contraction accelerates protein synthesis rates in adult feline cardiocytes.” The American journal of physiology 265, no. 2 Pt 2 (Aug 1993): H666-74.
Ivester, C T, W J Tuxworth, G Cooper, and P J McDermott. “Contraction accelerates myosin heavy chain synthesis rates in adult cardiocytes by an increase in the rate of translational initiation.” The Journal of biological chemistry270, no. 37 (Sep 1995): 21950-7.
Kato, S, C T Ivester, G Cooper, M R Zile, and P J McDermott. “Growth effects of electrically stimulated contraction on adult feline cardiocytes in primary culture.” The American journal of physiology 268, no. 6 Pt 2 (Jun 1995): H2495-504.
Ke, Lei, et al. “Calpain mediates cardiac troponin degradation and contractile dysfunction in atrial fibrillation.” Journal of Molecular and Cellular Cardiology 45, no. 5 (Nov 2008): 685-93.
Krüger, Martina, Christine Sachse, Wolfram H Zimmermann, Thomas Eschenhagen, Stefanie Klede, and Wolfgang A Linke. “Thyroid hormone regulates developmental titin isoform transitions via the phosphatidylinositol-3-kinase/ AKT pathway.” Circulation Research 102, no. 4 (Feb 2008): 439-47.
Kuramochi, Yukio, Chee Chew Lim, Xinxin Guo, Wilson S Colucci, Ronglih Liao, and Douglas B Sawyer. “Myocyte contractile activity modulates norepinephrine cytotoxicity and survival effects of neuregulin-1beta.” American journal of physiology Cell physiology 286, no. 2 (Feb 2004): C222-9.
Kuramochi, Yukio, Xinxin Guo, Douglas B Sawyer, and Chee Chew Lim. “Rapid electrical stimulation induces early activation of kinase signal transduction pathways and apoptosis in adult rat ventricular myocytes.” Exp Physiol 91, no. 4 (Jul 2006): 773-80.
Luiken, J J, J Willems, G J van der Vusse, and J F Glatz. “Electrostimulation enhances FAT/CD36-mediated long-chain fatty acid uptake by isolated rat cardiac myocytes.” Am J Physiol Endocrinol Metab 281, no. 4 (Oct 2001): E704-12.
Mills, Geoffrey D, Hajime Kubo, David M Harris, Remus M Berretta, Valentino Piacentino, and Steven R Houser. “Phosphorylation of phospholamban at threonine-17 reduces cardiac adrenergic contractile responsiveness in chronic pressure overload-induced hypertrophy.” Am J Physiol Heart Circ Physiol 291, no. 1 (Jul 2006): H61-70.
Nedachi, T, H Fujita, and M Kanzaki. “Contractile C2C12 myotube model for studying exercise-inducible responses in skeletal muscle.” American Journal of Physiology- Endocrinology And Metabolism, Jan 2008.
Nedachi, T, H Hatakeyama, T Kono, M Sato, and M Kanzaki. “Characterization of contraction-inducible CXC chemokines and their roles in C2C12 myocytes.” Am J Physiol Endocrinol Metab, Jul 2009.
Okutsu, S, H Hatakeyama, M Kanazaki, and H Tsubokawa. “Electric Pulse Stimulation Induces NMDA Glutamate Receptor mRNA in NIH3T3 Mouse Fibroblasts.” The Tohoku Journal of Experimental Medicine, Jan 2008.
Qi, X. Y, et al. “Cellular Signaling Underlying Atrial Tachycardia Remodeling of L-type Calcium Current.” Circulation Research 103, no. 8 (Oct 2008): 845-854.
Rana, O, E Saygili, C Meyer, C Gemein, and A Krüttgen …. “Regulation of nerve growth factor in the heart: The role of the calcineurin–NFAT pathway.” Journal of Molecular and Cellular Cardiology, Jan 2008.
Saeko. “Microsoft Word – 博士論文0627奥津_提出_.” Jun 2008: 1-21.
Schroder, Elizabeth, Miranda Byse, and Jonathan Satin. “L-type calcium channel C terminus autoregulates transcription.” Circulation Research 104, no. 12 (Jun 2009): 1373-81.
Thiel, W. H, et al. “Proarrhythmic Defects in Timothy Syndrome Require Calmodulin Kinase II.” Circulation 118, no. 22 (Nov 2008): 2225-2234.
Yamada, Masahisa, et al. “Electrical stimulation modulates fate determination of differentiating embryonic stem cells.” Stem Cells 25, no. 3 (Mar 2007): 562-70.