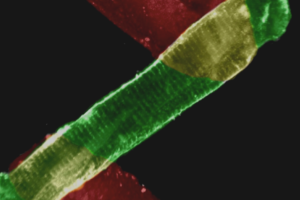
Hypertension, a major cardiovascular risk factor and cause of mortality worldwide, is thought to arise from primary renal abnormalities. However, the etiology of most cases of hypertension remains unexplained. Vascular tone, an important determinant of blood pressure, is regulated by nitric oxide, which causes vascular relaxation by increasing intracellular cGMP and activating cGMP-dependent protein kinase I (PKGI). Here we show that mice with a selective mutation in the N-terminal protein interaction domain of PKGI{alpha} display inherited vascular smooth muscle cell abnormalities of contraction, abnormal relaxation of large and resistance blood vessels, and increased systemic blood pressure. Renal function studies and responses to changes in dietary sodium in the PKGI{alpha} mutant mice are normal. These data reveal that PKGI{alpha} is required for normal VSMC physiology and support the idea that high blood pressure can arise from a primary abnormality of vascular smooth muscle cell contractile regulation, suggesting a new approach to the diagnosis and therapy of hypertension and cardiovascular diseases.
- HOME
- PRODUCTS
As a company of scientists, IonOptix is passionate about providing our colleagues with innovative solutions for high-speed quantitative fluorescence, muscle mechanics and tissue engineering research.
Browse by System, Software, Components or Research Application.
- RESOURCES
Browse our complete resource library including webinars, publications, user manuals, brochures and more.
Here customers can access software downloads and update their customer profile.
Need help? No problem! Connect with an IonOptix staff member by submitting a ticket.
- NEWS & EVENTS
- ABOUT
- CONTACT US
- Customer Login