SOLUTIONS FOR STEM CELL-DERIVED CARDIOMYOCYTES
Over the last decade human induced pluripotent stem cells (hiPSCs), as well as human embryonic stem cells (hESCs), have emerged as a model system complementary to classic approaches. These cells offer important advantages over typical adult ventricular cardiomyocytes, the most significant of which owing to their human genetic background. Despite their advantages, they are less mature, more amorphous, and provide poorer contrast. To broaden the utility of our products, we have developed several contrast-based algorithms within IonWizard, collectively called CytoMotion, to acquire contractility data from immature cells that lack refined structure and precise features.
Summary
There is a growing need for precise characterization of function in hiPSC-CMs. While several products exist on the market already, IonOptix is an established leader in the field and is known for precise, high-speed, real-time measurements of contractile kinetics dating as far back as the early 1990s with our Edge Detection algorithm for cell length data. To meet this growing demand for characterization of contractility parameters in hiPSC-CMs, we have implemented approaches that rely on variations in pixel intensity over time, collectively referred to as CytoMotion. The algorithm works for measurements made on both standard Calcium and Contractility as well as MultiCell Systems.
Scientific Reason for the Product
iPS cells, or induced pluripotent stem cells, derive from de-differentiated adult cells (reprogrammed dermal fibroblasts, blood mononuclear cells, etc.). This is significant in that the cells can be obtained from normal human tissue, giving the cells a human genetic background. Once de-differentiated, they can then be programmed to become “cardiomyocytes”, aka human-induced pluripotent stem cell cardiomyocytes or hiPSC-CMs. Other model systems such as adult rat and mouse CMs suffer from poor translation to human physiology (estimates vary, but one report states that ~80-85 % of drugs effective in mice are ineffective in humans and more than 30 % of drugs passing animal tests for safety show toxicity in human trials). An important distinction between animal model systems and human cardiomyocytes is the expression of the human ether-a-go-go-related gene (hERG), the pore-forming subunit of the rapidly activating delayed rectifier potassium channel (IKr) which is responsible for membrane repolarization. Perturbations to hERG expression and/or IKr activity is typically arrhythmogenic, making it critical to cardiotoxicity studies. Given their human genetic background, hiPSC-CMs offer long-term potential as a model system, such as:
- Modeling human heart function and pathophysiology (from disease and injury)
- Drug discovery (treating heart disease)
- Evaluating pharmaceutical toxicity pre-clinic (are candidate drugs likely to interfere with normal heart function)
- Tissue regeneration and native transplants (a long-term goal would be engineering tissue to replace damaged tissue without host tissue rejection)
While the use of hiPSC-CMs shows promise, there are also several limitations that make it difficult to get reliable, reproducible functional data, limiting the use of standard edge detection to characterize them:
- Immature
- Typically lack the rod-shaped morphology consistent with adult CMs
- Primitive E-C coupling (cannot always be stimulated)
- Inconsistent
- Morphology and phenotype may vary from lab to lab, even when using cells from the same source
- Poor Contrast
- Unlike adult CMs, hiPSC-CMs are often grown in a two dimensional layer, known as a monolayer, where the cells are mechanically attached to one another and gap junction-coupled; this obscures the contrast at cell peripheries.
- hiPSC-CMs are typically much smaller than freshly isolated primary cardiomyocytes. This does not pose an additional challenge in the x- and y-dimensions, but their lack of depth in the z-axis reduces their contrast.
To facilitate measurement of contractility in these challenging cells, we have developed a suite of contrast-based tools to acquire hiPSC-CM contractility, enabling analysis of the following critical parameters:
- Max contraction velocity
- Time to max contraction velocity
- Shortening time (time to percent peak)
- Max return velocity
- Time to max return velocity
- Relaxation/relengthening time (time to percent baseline)
- Beat frequency with variance (requires CytoSolver)
- Transient duration
IonOptix Systems for the study of stem cell-derived cardiomyocytes include…
MULTICELL SYSTEM
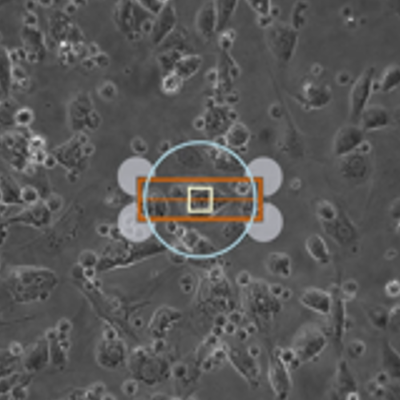
New for 2017, our MultiCell System offers fully-automated identification and mechanical phenotype quantification from hundreds of myocytes per hour without sacrificing any of the detailed and thorough analysis of our standard systems. Featuring a fast x-y-z position-programmable scanning microscope coupled with a novel image analysis method quantifying position, size, and orientation, MultiCell brings dynamic characterization of contraction-relaxation function into the 21st century with greater statistical power, better acquisition and analysis, and a more user-friendly design. Coupling MultiCell with our CytoMotion algorithm allows users to broaden the utility of the system beyond traditional isolated adult cardiomyocytes to hiPSC- and hESC-CMs. LEARN MORE
CALCIUM AND CONTRACTILITY SYSTEM
Our Contractility System and ever popular combination Calcium and Contractility System are cornerstones for reliable and repeatable quantification of cardiomyocyte contractile function. With the introduction of our CytoMotion algorithm, our Calcium and Contractility System can be used to characterize both adult myocytes and phenotypically immature myocytes, like hiPSC- and hESC-CMs. Providing important indicators of contraction/relaxation dynamics, as well as changes in calcium release and handling, our gold standard Calcium and Contractility System offers researchers the ability to fully characterize excitation-contraction coupling. LEARN MORE
CYTOMOTION LITE SYSTEM
Acquiring contractility kinetics in human induced pluripotent stem cells (hiPSCs) and human embryonic stem cells (hESCs) derived cardiomyocytes can be especially challenging given their amorphous phenotype and poorer contrast relative to adult ventricular cardiomyocytes. We’ve made it simple. Coupling our CytoMotion algorithm and IonWizard data acquisition with a high frame rate CMOS camera, CytoMotion Lite provides a low-cost, simplified solution for reliable data collection. LEARN MORE